
Research
Our Misson
The Dabertrand Lab seeks to understand how ion channels and calcium signaling regulate cerebral blood flow through pericytes, endothelial cells, and smooth muscle cells within the brain’s microcirculation. Current projects in the lab further examine the vascular contributions to cognitive impairment and dementia (VCID) in small vessel disease, Alzheimer’s disease but also how neurodevelopmental disorders can affect the cerebrovascular function.
Our Approach
Advanced myography: The regulation of cerebral blood flow is tightly controlled by changes in intracerebral arteriole and capillary diameter. To study these changes in real time, we have continuously developed innovative ex vivo imaging techniques of the mouse brain microcirculation. With ex vivo pressurized arterioles (diameter ~15 µm) isolated from different brain regions, e.g. cortex, amygdala, hippocampus, we can measure real-time constrictions and relaxations as well as changes in membrane potential in response to vasoactive agents. Furthermore, we are able to combine genetic expression of Ca2+ indicator in endothelial or smooth muscle cells with confocal microscopy to analyze intracellular Ca2+ signaling.
Spontaneous vasoconstriction in response to luminal pressure
Electrophysiology on freshly isolated cells and in vivo two-photon imaging allow us to further investigate the molecular mechanisms underlying cerebral blood flow regulation.
Isolated smooth muscle cell expressing Ca2+ indicator GCaMP6f
in vivo two-photon microscopy gives us access to the molecular mechanisms underlying cerebral blood flow regulation.
Two-photon imaging of the mouse brain vascular network. Cerebral blood flow regulation
Innovation
Our lab continuously adopts and develops innovative approaches to further understand how vascular health contribute to brain function. By integrating state-of-the-art technologies such as advanced imaging, molecular profiling, and computational modeling, we explore the complex interactions between the cerebral vasculature and neural activity and how disruptions in these processes impact brain health and contribute to neurological disorders. Through innovative methodologies, we aim to bridge the gap between fundamental vascular biology and its implications for cognitive function, aging, and disease, ultimately driving forward new therapeutic strategies for maintaining brain health.
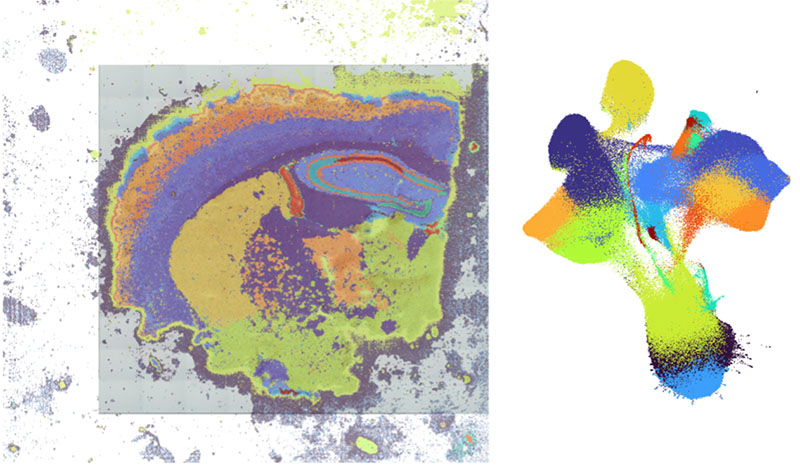
Single-cell resolution spatial transcriptomics enables our lab to map gene expression profiles to their precise locations within brain slices or isolated microcirculation at a single-cell level. This emerging technology offers transformative insights into cellular organization, tissue architecture, and the spatial relationships between cells in complex biological systems. Analysis by Eric Prince.
Main areas of research
Neurovascular Coupling
Local cerebral blood flow is precisely regulated by neurovascular coupling to ensure that neuronal metabolic demands are satisfied. Vasodilation of parenchymal arterioles is thought to mediate a large part of the response due to their ability to dilate rapidly in response to endogenous substances. In a seminal study conducted with Dr. Longden (presently at the University of Maryland) in Dr. Mark Nelson’s lab at the University of Vermont, we provided unequivocal evidence that brain capillaries act as a sensory web to translate neural activity into blood flow. We demonstrated a central role for capillary endothelial cells in sensing neural activity and communicating it to upstream arterioles in the form of an electrical vasodilatory signal. We found that this signal is initiated by extracellular potassium—a byproduct of neural activity—which activates capillary endothelial cell KIR2.1 channels to produce a rapidly propagating retrograde hyperpolarization that causes upstream arteriolar dilation, increasing blood flow into the capillary bed. To enable these experiments, we developed a novel ex vivo pressurized capillary-parenchymal arteriole (CaPA) preparation that allows the study of conducted electrical signaling from the capillaries to the upstream arteriole. Computational modelling with our collaborator Dr. Tsoukias further validated our model.
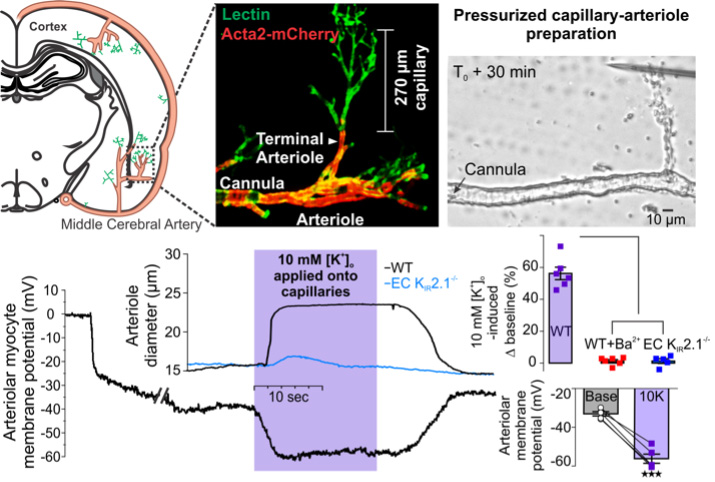
Capillary-to-arteriole electrical signaling
Brain capillaries act as a neural activity-sensing network wherein capillary endothelial cells (cECs) initiate an electrical (hyperpolarizing) signal in response to neural activity that rapidly propagates upstream to dilate feeding parenchymal arterioles and locally increase blood flow.
Prostaglandin E2 (PGE2), a metabolite of arachidonic acid, has also been proposed as a major dilatory agent mediating neurovascular coupling, despite evidence that it directly constricts arterioles. We resolved this controversy by showing that PGE2 can cause upstream arteriole dilation when applied onto capillaries. This new signaling modality posits a central role for G protein-coupled receptors of the Gq/11subtype—providing a paradigm-shifting view that nonetheless remains coherent with the broad contours of a substantial body of existing literature.
Capillary stimulation with PGE2 1 μM evokes upstream arterial dilation in ex vivo CaPA preparation.
Pathogenesis of CADASIL, an archetypal monogenic form of small vessel disease of the brain.
Using the aforementioned approaches in mouse brain microcirculation allows us to use transgenic mouse models for different conditions such cerebral small vessel disease, Alzheimer’s disease, hypertension and more recently, autism spectrum disorders, to investigate the pathomechanisms at play. In particular, our long-term transatlantic collaboration with Dr. Anne Joutel (Paris, France) has focused on the CADASIL syndrome, a hereditary form of small vessel disease caused by mutations in the NOTCH3 receptor. Cerebral small vessel disease is responsible for more than 25% of ischemic strokes and is the leading cause of age-related cognitive decline and dementia after Alzheimer’s disease. At the capillary level, we have found that CADASIL is associated with reduced synthesis of the phospholipid PIP2, which prevents the Kir2.1 channel-initiated capillary-to-arteriole electrical signaling that supports vasodilatory responses during functional hyperemia (see Neurovascular coupling above). We further showed that systemic injection of exogenous PIP2 is sufficient to rescue this deficit in CADASIL mice, restoring adequate cerebral blood flow in response to neuronal activation (patent number 62/823,378; “Methods to promote cerebral blood flow in the brain”). We are actively carrying on this finding to develop a chronic PIP2-based treatment and measure the impact of neurovascular deficits on cognitive functions.
Exogenous application of PIP2 fully restores upstream arterial dilation in response to capillary stimulation with 10 mM K+ in CADASIL mice.
Pericytes, the last line of cerebral blood flow regulation
The last decade has seen a dramatic upsurge in research on pericytes, the mural cells that wrap around capillary endothelial cells, with a focus on their role regulating cerebral blood flow in both health and disease. Because of technical challenges, several questions regarding their reactivity to blood pressure and the contractile pathways in place have been left unanswered. Through our pioneering work on capillaries-to-arteriole signaling, we have extended our ability to image brain microcirculation to the pericyte level not only in vivo, but also ex vivo.
Isolated brain arteriole with capillaries. Green shows smooth muscle cells and pericytes expressing the Ca2+ indicator GCaMP6f. Red shows AF 633-hydrazide elastin stain used to visually identify the presence of internal elastic lamina in arteriole, or its absence in capillaries.
Pericyte Ca2+ signals (green- GCaMP6f) and constriction in response to 100 nM U46619 visualized with TRITC (red) fluorescence.
GCaMP6f fluorescence reflecting cytosolic Ca2+ concentration increases and spontaneous Ca2+ signals in transitional pericytes in an ex vivo capillary-parenchymal arteriole preparation pressurized at 20 mmHg (left side) and 40 mmHg (right side).
Current Major Funding to Dr. Dabertrand (PI/MPI)
2R01HL136636
Capillary control of cerebral blood flow, and its disruption in small vessel disease
RF1/R01NS129022
Rescuing neurovascular coupling to protect neuronal plasticity and cognition
RF1/R01NS140137
Brain capillary endothelial cell energetics and neurovascular uncoupling in dementia
Foundation Leducq Transatlantic Network of Excellence
Brain Endothelium: A Nexus for Cerebral Small Vessel Disease.
Ludeman Family Center for Women’s Health Research – Anschutz Medical Campus
Neurovascular origin of female protective effect in autism
To lab Members
F31HL170645 (Danielle Jeffrey – predoctoral award)
Pericyte KATP channel hyperactivity in cerebral small vessel disease
Dabertrand Laboratory | Department of Anesthesiology | University of Colorado Anschutz
Dabertrand Laboratory
Department of Anesthesiology
University of Colorado